The South West genomic Laboratory Hub is committed to providing a service of the highest quality.
Information on accreditation, quality assurance including turn around times, user feedback and complaints procedures can be accessed below.
Accreditation
Bristol Genetics Laboratory (BGL) and Exeter Genetics Laboratory (EGL) are UKAS accredited medical laboratories numbers 9307 and 8092. UKAS assesses against the International Standard for medical laboratories, BS EN ISO15189:2012. Further information on accreditation is available via the UKAS website.
Details of activities that are currently provided under the scope of accreditation can be found at:
- BGL Schedule of Accreditation
- EGL Schedule of Accreditation - link to follow
In certain circumstances laboratory tests will be performed outside the scope of accreditation. This may arise when new services are introduced, for which UKAS accreditation is yet to be awarded.
Application for extension to the laboratory scope will be submitted, however, whilst accreditation is pending the test continues to be performed to the same rigorous internal quality control standards and protocols as accredited tests.
It should be noted that elements of the Whole Genome Sequencing service are undertaken by an external provider and therefore are provided outside the laboratory’s UKAS scope of accreditation.
Methods currently pending accreditation are as follows:
| Activity | Comments |
|---|---|
| Illumina Novaseq X Plus sequencer used in Next Generation Sequencing (NGS) | |
| Next Generation Sequencing (NGS) using Roche solid tumour and haemato-oncology RNA panels | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Myra Liquid Handling Robot used in preparation of NGS and digital droplet PCR assays | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Whole Genome Sequencing sample preparation, analysis and reporting | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Homologous Recombination Deficiency (HRD) service analysing shallow WGS data through SOPHiA DDM “GIInger” pipeline | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Scanning of MethylationEPIC Arrays for central nervous system tumours using Illumina iScan | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Use of Azure 280 for FSHD1 southern blotting image capture | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Oncology MSI-Plus NGS service | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Univ8 EuroClonality NGS DNA capture panel for MRD | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Illumina GSA Cyto SNP microarray and analysis using Bionano VIA software enterprise | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Extraction of DNA from saliva and buccal brush samples using the EZ1 Biorobot | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Automation of preparatory steps to extraction of DNA and RNA from FFPE using Promega MaxPrep | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| MDM2 amplification testing by Digital Droplet PCR (ddPCR) | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Gene Amplification Detection on the RMH200ST Solid Tumour Assay and Pipeline | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
| Gene screening of large gene panels for genetic variants using the DNAnexus Rare Disease pipeline | Application to UKAS for extension to scope of accreditation submitted. Pending assessment. |
External Quality Assessment
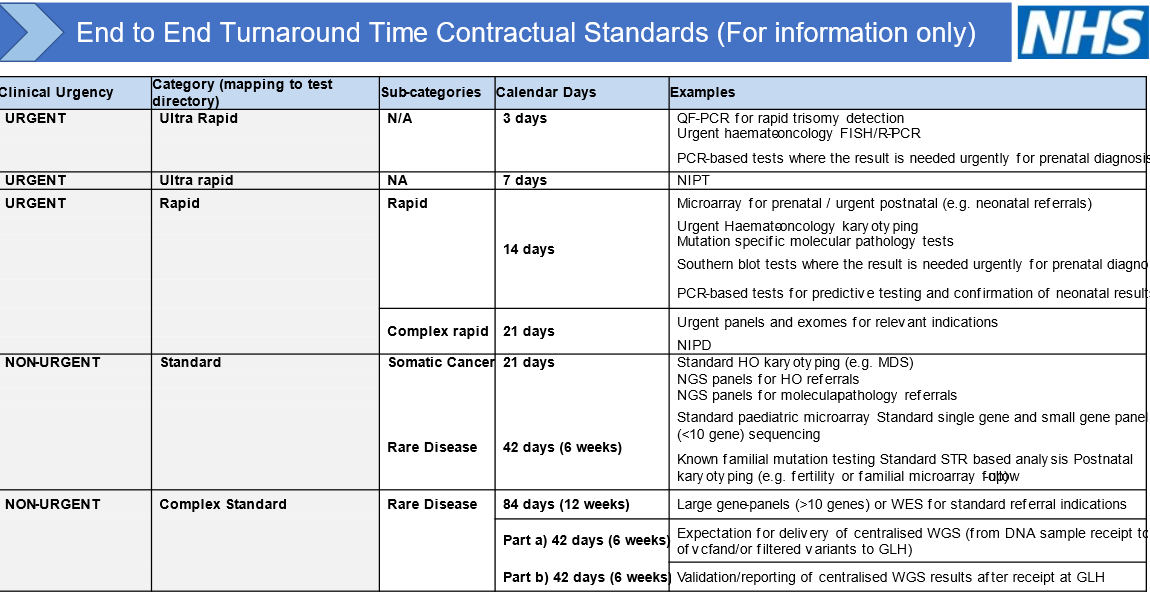
Target Turnaround Times
The SWGLH is working towards 90% compliance with national reporting time guidelines stated in the table below:
User Feedback
As the SWGLH, we strive to fulfil the requirements of all our service users and conduct regular user surveys.
We are always happy to receive comments and feedback from our service users. If you would like to provide feedback please email us at SWGLHenquirie@nbt.nhs.usk.
This drives continual improvement and ensures that our service meets the needs of both referrers and patients.
Complaints
We would like to assure our service users that complaints are taken very seriously and are fully investigated.
The SWGLH laboratories respond to complaints in line with their respective Trust complaints policies and procedures.
Further information on the procedures can be found via the following links:
This page was last updated 09/02/2026
