What is a chest drain?
A chest drain is a narrow tube that is inserted between the ribs and sits in the space between the lung and the chest wall. This space is lined on both sides by a membrane called the pleura and is known as the pleural cavity or pleural space.
A chest drain is inserted when air, fluid, blood or pus has collected in the pleural space.
The external end of the chest drain tube is attached to a bottle which acts as a seal to prevent anything from leaking back into the pleural space.
What is a chest drain for?
You need a chest drain if you have an air leak (pneumothorax), a collection of fluid (pleural effusion), pus (empyema) or blood (haemothorax) in the pleural space. Any of these can cause problems with breathing and can stop the lungs from working properly. The chest drain will allow the fluid or air to leave the body and allow your lung to re-expand.
How does a chest drain work?
Once a chest drain has been inserted the external end is connected to a bottle. The fluid or air travels down the tube, into the bottle.
There are 2 types of chest drain bottle. The first type contains a small amount of water, which acts as a seal preventing air or fluid coming back up the tube into your chest. Alternatively, an electronic chest drainage device may be used (a Thopaz machine), which can deliver suction (to help the lung re-expand) and allows the doctors to measure if there is any ongoing air leak between the lung and pleural cavity.
Before the chest drain is inserted
Before the chest drain is inserted, the doctor will discuss the procedure with you and you will have an opportunity to ask questions. You will then need to sign a consent form.
Blood tests may be needed before the procedure.
If you are on blood thinning medication, they may need to be stopped before the chest drain is inserted, to minimise the risk of bleeding during the procedure.
Please let the doctor know if and why you take blood thinning medication and they will make a plan with you about when it should be stopped.
There are potential rare complications of stopping blood thinning medications but these are generally very rare and the risk is lower than the possible bleeding risk if they are continued.
How will the chest drain be put in?
- You will either sit with your head and arms resting on a pillow on a table or lie on your bed with your arm above your head. The drain is usually put into the side of your chest below the armpit.
- Before the procedure starts, an ultrasound may be performed to choose the best place to insert the drain. An ultrasound is painless and a cool gel is used on the skin to ensure good contact for the ultrasound tip. You may be offered painkillers to take before the procedure starts.
- The procedure is performed using an aseptic technique to minimise the risk of infection. Your skin is cleaned with an alcohol-based liquid to kill any bacteria. A local anaesthetic is then injected into the skin to numb the area where the tubeis to be inserted, which can ‘sting’ temporarily but resolves quickly. A small cut (approximately 2-3mm) is then made in the anaesthetised area. It is normal to feel a sensation of pressure and tugging as the drain is gently eased into the chest, but this should not be painful.
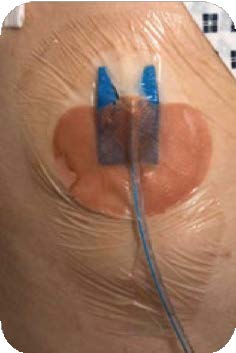
- The chest drain is held in place with stitches and the exit site is covered with a waterproof dressing. The end of the tubing is connected to a drainage bottle.
- Your chest drain will be monitored regularly. You may be asked to cough, or take a deep breath. This enables the doctor or nurse to ensure the drain is still functioning. You will be given regular pain relief while the drain is in place. Pain may impair your movement and breathing which may prolong the time your lung takes to expand therefore it is important to report any pain and keep it under control.
The bottle to which the chest drain tubing is attached
The bottle contains a small amount of water, which acts as a seal preventing air or fluid coming back up the tube into your chest.
The bottle to which the chest drain tubing is attached
The bottle must be kept below the level of the waist at all times.
Insertion site of the drain into the chest
It is secured with a stitch and a waterproof dressing is placed over the top.
Thopaz machine (an electronic chest drain device)
Your drain may be attached to this machine, rather than to a bottle, if you have a pneumothorax (air leak into the pleural space). This allows the air leak to be accurately measured and can be used to apply gentle suction to the drain.
Looking after your chest drain
As the fluid or air around the lung drains you should be able to move more easily. There are a few simple rules that you can follow to minimise any problems:
- You can move and walk around with a chest drain but you must remember to carry the drainage bottle with you.
- Always carry the bottle below the level of your waist. If it is lifted above your waist level fluid from the bottle may flow back into the pleural space.
- Whilst in bed keep the drainage bottle on the floor.
- Don’t pull on your chest drain or tangle it around your bed.
- Do not swing the bottle by the tube.
- Try not to knock the bottle over.
- If your chest is painful please tell your nurse.
- If you feel your tube may have moved or may be coming out please tell your nurse.
- Inform your nurse if you feel any increased shortness of breath.
When is the drain taken out?
How long the chest drain will be needed depends on your condition and how well you respond to treatment.
Removing the drain is a simple procedure. Once all the dressings are removed, the stitch is cut and the drain is gently pulled out. The doctor or nurse may ask you to breathe in a particular way while the drain is removed. Removal of the drain can feel a little uncomfortable but only lasts a few seconds. You will have a chest X-ray once the drain has been removed.
In some cases a stitch is left where the drain has been. This needs to be removed after 7 to 10 days.
If you experience discomfort after the drain has been taken out you can take simple painkillers. If you develop any other worsening symptoms (lots of pain, difficulty breathing or a temperature) you must tell the doctors and nurses.
Are there any risks with chest drains?
In most cases the insertion of a chest drain is a routine and safe procedure. Most people find their breathing is much easier once the chest drain is in place. However, like all medical procedures, chest drains can cause some problems:
- Chest drains sometimes fall out and may need to be replaced (this happens in less than 1 in 10 drains). This risk is minimised by stitching the drain in place and covering it with a secure dressing. You can also help by following the suggestions above (‘Looking after your chest drain’).
- Fewer than 1 in 10 drains may become blocked, which stops them working effectively.
- You may feel temporarily dizzy or light-headed when the drain is inserted. This occurs in about 1 in 50 patients. It is usually short-lived, but please let your doctor know if you experience this.
- Pain: most people (1 in 2 people having a drain) experience some discomfort from their chest drain but painkiller medication should control this.
- Infection: sometimes chest drains can become infected but this is uncommon (about 1 in 50 patients). Thorough cleaning of the skin before putting in the chest drain and a good aseptic technique will help to reduce this risk. If you feel feverish or notice any increase in pain or redness around the chest drain, inform your nurse or doctor.
- Bleeding: a bruise at the site of insertion occurs commonly. Rarely (less than 1 in 250 patients), the chest drain may accidentally damage a blood vessel and cause bleeding into the pleural cavity. Often it stops by itself but occasionally it might require an operation or other intervention to stop the bleeding.
- Subcutaneous emphysema: sometimes air can collect under the skin near the chest drain, causing swelling or a ‘crackly’ feeling (less than 1 in 25). Usually this resolves by itself, but occasionally may require a larger drain to be inserted.
- Re-expansion pulmonary oedema: if the lung re-expands quickly, there is a risk of fluid collecting in the lung itself, which can occur in around 1 in 200 patients. This can cause a cough or worsening breathlessness. If you experience these symptoms, please let the nursing or medical team know as soon as possible as it may help if the fluid is drained more slowly.
- Chest drain wrongly positioned: if the drain is being inserted for fluid in the pleural cavity, an ultrasound will be used to guide where the drain is placed to help position the drain correctly. However in about 1 in 50 patients, the tip of the drain is not placed in the pleural cavity and instead sits in the tissues of the chest wall. If this is the case, a new drain may need to be inserted.
- A very rare complication of a drain insertion (about 1 in 200) is puncture of another organ. This could include other structures in the chest (e.g. the lung, heart, diaphragm or major blood vessel) or abdominal organs (e.g. stomach, liver or spleen). If the drain punctures the underlying lung, it may require the drain to stay in place longer. If organ puncture were to happen, another procedure or operation may be needed.
- Death: less than 1 in 1000 risk.
References and further information
If you require further information, please speak to your doctors and nurses.
Hooper et al., Pleural procedures and patient safety: a national BTS audit of practice. Thorax 2015; 70:198-191.
How to contact us
Brunel building
Southmead Hospital
Westbury-on-Trym
Bristol
BS10 5NB
0117 414 6337
If you or the individual you are caring for need support reading this leaflet please ask a member of staff for advice. If you’re an overseas visitor, you may need to pay for your treatment or you could face fraud or bribery charges, so please contact the overseas office: