Angiogram, angioplasty, and stents
There are two types of treatment to open up blocked or narrowed arteries, without the need to make an opening in the skin. These are called “angioplasty” or “stenting,” and can be done using X-ray guidance. They are usually performed while you are awake, using local anaesthetic to numb the skin.
What is an angiogram?
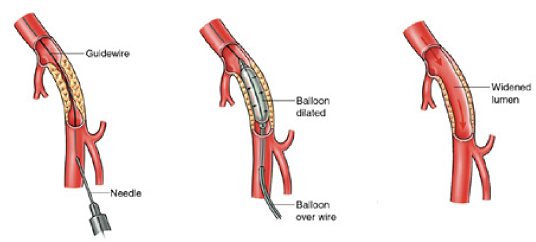
The first stage of your procedure is called an angiogram. This is used to make a detailed map of your arteries, because blood vessels do not show up on ordinary X-rays. A needle, followed by a thin plastic tube (catheter) is inserted into your artery to do this.
What is an angioplasty?
After the angiogram, a soft guidewire is inserted into the artery through the catheter. The wire must then be passed through the blockage or narrowed section of artery, if this is not possible we will discuss your options with you. A special balloon is then passed over the top of the wire. This balloon is inflated to stretch open the blockage or narrowing and allow more blood to flow through.
Once the artery has been stretched the balloon is removed and another angiogram is done to look at the result. If it is not possible to pass through the blocked artery we will discuss your options with you.
What is a stent?
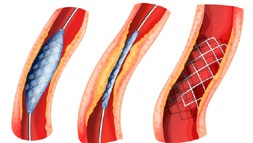
A stent is a hollow mesh tube about the size of your little finger. It is made of fine, sterile metal and is used to open an artery. When it is used, unlike an angioplasty balloon, it is left inside the artery after the procedure and remains there forever. Once it is in position the stent will not give you any discomfort or sensation. It is important to remember it is there and to tell any doctors treating you in the future that you have it. It can be placed in the artery using similar equipment to the angioplasty and usually requires no additional anaesthetic.
Stents are often used if there is a long blockage in a large artery. It may also be used if you have an angioplasty and the result is not as good as hoped for, or if the artery is not smooth enough for an angioplasty.
If with time, a narrowing that was treated with an angioplasty reoccurs, a stent can be used to decrease the likelihood of it narrowing again. Narrowing can also develop within or around a stent, or the stent could get blocked. If the stent blocks, your symptoms may return or get worse.
Where will the procedure be done?
Most angioplasties/stents are performed by the Major Arterial Centre in Southmead Hospital. They are done by specialist radiology doctors called interventional radiologists, who are part of the multidisciplinary team involved in treating patients with vascular disease. Unless you live alone, are frail, or have certain other medical needs, you will usually go home the same day as your procedure.
The procedure is done in a specially adapted room called an “Interventional Radiology (IR) room”. Some patients will have surgery at the same time as angioplasty or stenting. This is done in a specialist operating theatre (hybrid theatre) which has X-ray equipment.
Who will be doing the angiogram or angioplasty?
The procedure will be performed by a team of specialists. In most cases the team is led by a consultant interventional radiologist. The lead doctor may be assisted by another specialist, or a doctor training in radiology or vascular surgery. Other members of the team in the IR room will include radiology nurses and radiographers.
How do I prepare for an angiogram?
Before the procedure
- There is no preparation for this procedure; you can eat and drink as normal unless we tell you otherwise.
- If you take blood thinning tablets (including anti-platelets) please contact the Imaging Department before your appointment using the phone number on your appointment letter as they may ask you to speak to your GP.
- Please inform us if there is any possibility you are pregnant.
- Please make arrangements for someone to collect you from the hospital after your procedure and take you home by car. We advise you do not use public transport. For the first 24 hours following your procedure you are not permitted to drive, and we advise someone stays with you at home. Please tell the Imaging Department if this is not possible so we can make alternative arrangements.
On the day of the procedure
- You will arrive at the Imaging Department (Gate 19) and will be accompanied into our day case area.
- Please bring a list of your regular medications with you.
- Please inform us if you are allergic to anything.
- You may take your normal medication unless instructed otherwise.
- An interventional radiologist will discuss the procedure with you including the benefits and potential risks to you. You will have an opportunity to ask questions about the procedure. If you choose to have the procedure you will need to sign a consent form.
- You will be asked to change into a hospital gown.
- We will place a small plastic tube (cannula) into a vein in your arm or hand – this allows us to administer medications or intravenous fluids during the procedure if necessary.
- Once all the checks have been performed and consent form signed, you will be taken to the IR room. You will be asked to lie down on the X-ray table for the procedure. There will be a small team of nurses, doctors, and radiographers with you throughout.
- Monitoring equipment will be attached to you so we can monitor your blood pressure, heart rate, and oxygen levels throughout the procedure.
- The nurse will then clean the area at the top of your leg with an antiseptic solution and cover you with sterile drapes. The X-ray machine at this point may move around you but will not touch you.
- An ultrasound machine will be used to find a suitable blood vessel.
- The interventional radiologist will then inject local anaesthetic into the area at the top of your leg, which may briefly sting and then go numb. After this, you may just feel a pushing sensation when a small plastic tube (catheter) is inserted into your artery and the catheter fed through.
- Once the catheter is moved into the correct position, contrast medium (X-ray dye) will be injected into different blood vessels and X-ray images are taken. The injection of the contrast medium, may give you a momentary warm feeling. n The injection will be repeated until all the necessary images have been obtained.
- The interventional radiologist will then usually discuss the map of your arteries with you and go through the pro and cons of proceeding with treatment to open up the blocked or narrow segments.
- Sometimes the interventional radiologist may find that the pattern of artery disease is different to what we were expecting. It may be that that the risks of doing a procedure under X-ray guidance to improve the circulation are not advisable. This will be discussed with you.
- At the end of the procedure the catheter will be removed from your leg.
- Usually one of the team will press on the area where the catheter was inserted for 10-15 minutes to prevent any bleeding. If more appropriate, the specialist will use a plug or “closure device” to seal up the artery where the catheter was inserted.
Will the procedure hurt?
When the local anaesthetic is injected it will sting to start with, but this soon wears off. The skin and deeper tissues should then start to feel numb. After this the procedure should not be painful but you may feel pressure or pushing. If the procedure becomes uncomfortable we can give you some painkillers through the cannula in your arm.
As the contrast medium passes around your body you may get a warm feeling, which some people find a little unpleasant. This will soon pass and should not concern you.
Some people feel a bit of discomfort when the angioplasty balloon is inflated or when the stent is expanded. This usually passes quickly when the balloon is removed.
How long will it take?
Every patient’s situation is different, and it is not always easy to predict how straightforward the procedure will be. For example, those with a large artery in the leg are usually straightforward and do not take long – around half an hour. Other times the vessels may be much smaller and complex, and the procedure can take 2-3 hours.
It is important to be aware of rare, but serious complications:
- Bleeding can occur from the place where the catheter entered the artery in the groin. This may result in a large, painful bruise (haematoma) which requires you to stay in hospital and have surgery. The occurs in: angiogram: 3 in 100 patients; angioplasty/stent: 4 in 100 patients.
- The artery may not seal up at all in the area where the catheter was inserted (a false aneurysm). This may happen in 1 in 500 patients. It may require a blood transfusion or further procedures including surgery.
- Sometimes it is not possible to cross the blockage in the artery. Occasionally the narrowing or blockage cannot be opened up, or the angioplasty/stent fails immediately. n
- Occasionally small fragments of blood clots break off and lodge in the artery below the angioplasty. This may require you to stay in hospital for close observation to take blood thinning medication, and/or have emergency surgery. n
- Occasionally the artery can rupture (leak) following the angioplasty. This can sometimes be treated in the Imaging Department by using a stent in the artery to seal the tear. If this is not possible an operation may be required.
Overall there is a 1 in 100 chance an angioplasty will fail and immediately make your leg worse. In this situation you may need urgent surgery. In severely diseased arteries the risk of requiring urgent surgery is 3 in 100. If urgent surgery fails to restore blood flow to the leg you may even need an amputation.
What problems can occur after this procedure?
Complications following an angioplasty are less frequent than following surgical alternatives but can include:
- Allergic reaction to the X-ray contrast medium. In most cases this is a minor reaction. Very rarely (1 in 1500) a reaction may be severe and need to be treated with medicines.
- X-ray contrast medium can affect kidney function. If you are at risk of this, special precautions will be taken to reduce the chances of problems occurring.
- A small risk from the X-rays used. The team will work hard to keep the dose as low as possible.
- Over time the treated section of the artery may narrow again. This can happen following both angioplasty or stenting. If you have a stent inserted into an artery, you may be referred for an ultrasound surveillance programme for a year after your procedure to detect this.
What will happen afterwards?
You will be taken back to our day case area on a trolley. Nurses in the day case area will carry out routine observations such as taking your pulse and blood pressure to make sure there are no problems. They will also look at your skin where the catheter was inserted to make sure there is no swelling or bleeding around it. In most cases you will be required to lay flat in bed for a few hours until you have recovered. If the specialist has used a closure device you will be able to sit up quite soon after your procedure.
Your specialist may make recommendations about your usual medication following the procedure. It is common to prescribe a short course (6 weeks) of tablets to help keep your angioplasty or stent open and working well whilst the artery repairs itself.
How soon will I recover?
If your procedure goes as planned, most patients come into hospital, and go home from hospital, on the same day.
If you go home the same day a responsible adult should accompany you home in a car or taxi. They should stay with you at home for 24 hours. You should not drive, operate machinery, or do any potentially dangerous activities for at least 24 hours. You should wait longer if you don’t feel fully recovered.
You should not do strenuous exercise for 1-2 days.
You should check your travel insurance if you wish to travel within 4 weeks of this procedure.
How effective is angioplasty or stenting?
The benefits you get from a successful procedure depends on many factors, especially whether you smoke, and the pattern of your arterial disease.
The results of angioplasty and stenting are most effective when they treat:
- Short sections of arterial disease.
- Narrowing rather than blockages.
- Large arteries like those higher in the leg such as thigh arteries rather than knee arteries.
- Patients who have single areas in the legs with narrowing/ blockages.
Is there anything I can do to help?
You cannot do anything to relieve the actual narrowing or blockage being treated.
You can improve your general health by doing regular exercise, stopping smoking, and reducing fat in your diet. These actions will help slow down the hardening of arteries which caused the problems in the first place, and may help you avoid the need for further treatment in the future.
When will I be followed up by the team?
Most patients will have a telephone follow-up with a clinical nurse specialist. This is a member of the specialist team.
If you have ongoing symptoms, we will arrange for you to be seen in an outpatients appointment.
If you have had a stent inserted in the thigh artery or behind the knee, the vascular team will arrange for you to have an ultrasound (arterial duplex) done at your closest hospital, around 6 weeks after your procedure. You will then be asked to attend further scans (surveillance) at regular intervals over the next 12 months. Sometimes the stent can develop narrowing without causing any symptoms. Your specialist would then discuss the pros and cons of intervention (further angioplasty).
We hope this information is helpful. If you have any questions before or after the procedure, the staff in the Imaging Department will be happy to answer them. The phone number is on your appointment letter.
Where can I find more information?
About vascular conditions and surgeries:
The Circulation Foundation | The UK Vascular Disease Charity
Patients and Referring Physicians | Society for Vascular Surgery
Home - Vascular Society
NICE | The National Institute for Health and Care Excellence
Peripheral arterial disease (PAD) - NHS (www.nhs.uk)
About our consultants:
A-Z of Consultants | North Bristol NHS Trust (nbt.nhs.uk)
Find a Vascular Society Member - Vascular Society
© North Bristol NHS Trust. This edition published January 2024. Review due January 2027. NBT003077.