Moving forward after breast cancer
Introducing supported self-management
North Bristol NHS Trust (NBT) has introduced supported self-management and remote monitoring. This is done using a website called My Medical Record (MMR). This can be used to manage your follow-up care securely.
Regular follow-up appointments can involve organising travel and time off work, which can be costly and inconvenient. Although some people find these appointments useful and reassuring, some find them unnecessary unless they have something specific to discuss.
If individuals report symptoms and concerns as they occur, rather than waiting for a routine appointment, this can help address concerns more quickly.
Following breast cancer treatment, you will be followed up for a period of time (usually 5 years) when you can contact your Breast Care Team directly if you have any queries or concerns.
What does supported self-management and remote monitoring mean?
- Supported self-management: This enables you to take a leading role in your follow-up with support as needed.
- Remote monitored: The Breast Care team can monitor your health and plan of care even when you are not in a face-to-face appointment.
The main aim is to enable you to develop the skills and knowledge to:
- Make positive choices about your health.
- Manage the physical and emotional impact of breast cancer and its treatment.
- Make healthy lifestyle changes.
Follow-up after breast cancer treatment
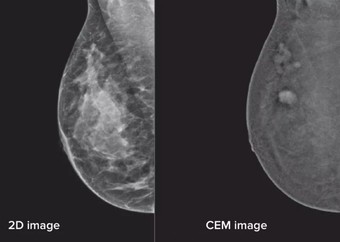
Depending on the type of treatment you have had, you may have a plan of regular mammograms or other imaging.
At NBT we use MMR to provide you with your monitoring plan, test results, and useful resources.
You can use it to message your cancer support team securely with non-urgent queries.
After finishing treatment, a Breast Clinical Nurse Specialist will discuss your monitoring plan and how self-supported management works.
Once introduced to MMR, you will be offered access to the website. You can access the website from your smart phone, tablet, or computer at any time.
Here is a short film about MMR:
After watching this video, if you would like to use MMR, you will be provided with login details. You can also choose not to use MMR, your monitoring will continue with routine telephone appointments.
What tests will I have?
- Yearly mammogram or MRI depending on your follow-up plan. We call this surveillance. Usually this is for 5 years.
- Depending on your treatment pathway, you may be prescribed anti-hormone tablets. We call this endocrine therapy.
- At the end of your monitoring period, you may continue to have imaging tests under the National Breast Screening Programme, depending on your age.
Imaging appointments will be arranged by the Radiology Administrative Team.
You will receive an appointment to attend the hospital typically 1 year from when you had your surgery, and yearly afterwards for the monitoring period.
Your mammograms will be carried out by the Radiology department at the Breast Care Centre during your period of follow-up.
MMR will show when future tests are due.
- If your test results are normal, a digital letter confirming this will be available on MMR, as well as the date of your next test.
- If your test results are abnormal, the Breast Cancer Team will contact you. You will likely be asked to come to an appointment at the Breast Care Centre.
Picture of the MMR homepage
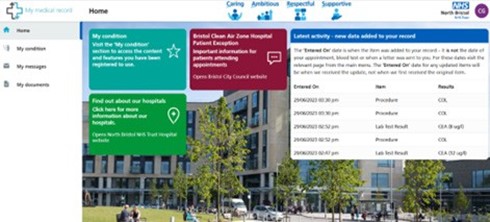
Useful resources available on MMR
Information resources including websites, leaflets and videos are available on MMR. These cover topics such as:
- Managing the side effects of treatment.
- Self-examination.
- Healthy lifestyle.
- Support groups.
Contact information
Breast Care Admin Team
- Queries relating to appointments.
- 0117 414 7000
Breast Care Support Workers
- Support and information relating to breast cancer.
- 0117 414 7047
Radiology Administrative Team
- Queries relating to mammograms and screening.
- 0117 414 9132
Macmillan Wellbeing Centre
- General support and information.
- 0117 414 7051
If there is no answer, please leave a message and we will get back to you as soon as possible. We will aim to get back to you within 48 hours.
© North Bristol NHS Trust. This edition published February 2026. Review due February 2029. NBT003691