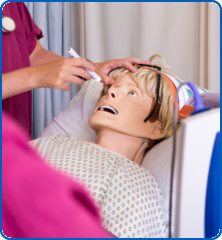
Our 'Train the Trainer' course provides a thorough introduction to using simulation based education for training healthcare staff.
The course is open to all healthcare professionals, suitable for anyone with an interest in simulation training.
A follow up 1/2 day now allows attendees to further practise the skills and knowledge gained on day 1 as they take part in the delivery of one of our 'Emergency' simulation sessions.
Topics covered include:
- Key education theories
- planning, preparing and running scenarios
- Using simulation manikins & simulation technology
- Group debriefing
- Support as faculty on 'Emergency' simulation course
Feedback from previous attendees:
"Very enjoyable and helpful course for anyone wanting to run simulations"
"This is an excellent beginners level course on planning and running simulation training"
"An excellent course that has inspired me to get more involved in developing and providing simulation sessions"
"Brilliant course, I will be recommending it to my colleagues and will use so much of what I learnt to apply to future simulations I run as a
clinical skills trainer"
"Extremely well delivered course that covers the essentials of what you need to embark on delivering simulation teaching sessions"
"Well executed, relevant to all grades, useful for my future practice as well as teaching others"
Course Fee: £195.00
Email SimSpace to book a place.