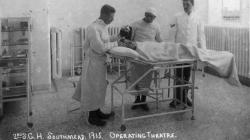
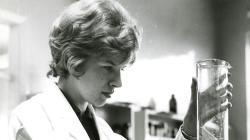
External Views
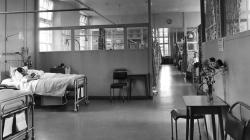
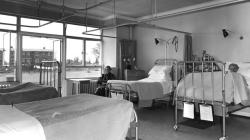
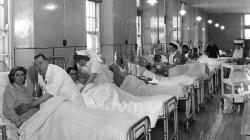
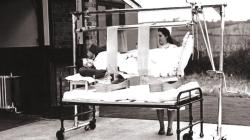
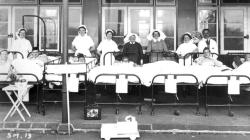
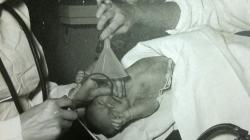
Internal Views
The Cranes of Southmead
Royal Visits
Royal Visits
Amendments
Changes that you make to your protocol and related documents after the project has been approved are called ‘amendments’.
Amendments may be substantial or non-substantial:
- Substantial amendments involve significant changes to the design and methodology of a research project.
- Non-substantial amendments do not involve significant changes to the design and methodology of a research project.
Definitions of substantial and non-substantial amendments
A substantial amendment is defined as an amendment to the terms of the application, or to the protocol or any other supporting documentation, that is likely to affect to a significant degree:
- The safety or physical or mental integrity of the subjects of the study.
- The scientific value of the study.
- The conduct or management of the study.
- The quality or safety of any investigational medicinal product used in the trial.
Examples of non-substantial amendments include:
- Minor changes to the protocol or other study documentation, e.g. correcting errors, updating contact points, minor clarifications.
- Updates of the investigator’s brochure.
- Changes to the chief investigator’s research team.
- changes to the research team at particular trial sites.
- Changes in funding arrangements.
- Changes in the documentation used by the research team for recording study data.
- Changes in the logistical arrangements for storing or transporting samples.
- Inclusion of new sites and investigators.
- Change to the study end date.
For all studies, it is the responsibility of the sponsor to determine whether an amendment is substantial.
A detailed list of substantial and non-substantial amendment examples can be found on the HRA website.
Changes to contact details for the sponsor (or the sponsor’s representative), chief investigator or other study staff are minor amendments but should be notified to the main REC for information.
View Our Research
Explore the ground-breaking research currently taking place at North Bristol NHS Trust.
About Research & Development

Find out more about our research and how we're working to improve patient care.
Contact Research
Research & Development
North Bristol NHS Trust
Level 3, Learning & Research building
Southmead Hospital
Westbury-on-Trym
Bristol, BS10 5NB
Telephone: 0117 4149330
Email: research@nbt.nhs.uk
Development Safety Update Reports
The Development Safety Update Report (DSUR), also known as the ctIMP Annual Safety Report is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are under further study) among the International Conference on Harmonisation (ICH) regions.
US and EU regulators consider that the DSUR, submitted annually, would meet national and regional requirements currently met by the US Investigational New Drug Annual Report and the EU Annual Safety Report, respectively, and will therefore take the place of existing safety reports.
The main objective of a DSUR is to present a comprehensive annual review and evaluation of pertinent safety information collected during the reporting period related to a drug under investigation, whether or not it is marketed, by:
- Examining whether the information obtained by the sponsor during the reporting period is in accord with previous knowledge of the investigational drug’s safety.
- Describing new safety issues that could have an impact on the protection of clinical trial subjects.
- Summarising the current understanding and management of identified and potential risks.
- Providing an update on the status of the clinical investigation/development programme and study results.
For international trials, reference on DSUR production and submission will be detailed in contractual agreements and/or specific protocol documents.
It is the responsibility of the sponsor to send our DSUR reports annually for every ctIMP study to the MHRA and to the main REC.
View Our Research
Explore the ground-breaking research currently taking place at North Bristol NHS Trust.
About Research & Development

Find out more about our research and how we're working to improve patient care.
Contact Research
Research & Development
North Bristol NHS Trust
Level 3, Learning & Research building
Southmead Hospital
Westbury-on-Trym
Bristol, BS10 5NB
Telephone: 0117 4149330
Email: research@nbt.nhs.uk