Early Inflammatory Arthritis (EIA)
We are developing our EIA service in line with NICE and BSR guidelines and around our patients’ needs.
All referrals are triaged by the consultants and we aim to see suspected early inflammatory arthritis cases within 3 weeks from receipt of referral. Referrals should be submitted with a completed proforma.
Please refer patients with suspected persistent joint inflammation of 4 weeks or more AND any one of the following:
- Swelling of 3 or more joints
- Swelling of the small joints of hands or feet
- Positive MCPJ or MTPJ “Squeeze test” (i.e. pain produced by squeezing across the metacarpophalangeal/etatarsophalangeal joints)
- Early morning joint stiffness (EMS) >30mins
Please note the diagnosis of Early Inflammatory Arthritis is not excluded by normal inflammatory markers and / or a negative rheumatoid factor and/or normal Xrays.
We aim to make a diagnosis and start disease-modifying drugs within 6 weeks from time of referral or discharge patients back if not EIA.
People diagnosed with EIA have regular clinical reviews by the multidisciplinary team for education, self-management, monitoring of disease activity, therapeutic benefit and treatment safety.
Referrals will only be accepted with a completed Referral pathway to the Early Inflammatory Arthritis Service (EIA Service) - 3 week wait attached to the eReferral.
Patients not meeting these criteria should be referred to general rheumatology clinics.
Please refrain from using steroids until the patient has been seen in clinic as this makes assessment very difficult.


 This is an operation for achalasia of the cardia, a condition in which the muscle in the lower oesophagus fails to relax and therefore hinders the passage of food and fluid into the stomach. Treatment with Botox injections or balloon dilatation of the affected part of the oesophagus can be attempted but are often short-lived and surgery is frequently required as a more lasting solution.
This is an operation for achalasia of the cardia, a condition in which the muscle in the lower oesophagus fails to relax and therefore hinders the passage of food and fluid into the stomach. Treatment with Botox injections or balloon dilatation of the affected part of the oesophagus can be attempted but are often short-lived and surgery is frequently required as a more lasting solution.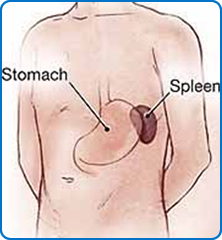 The spleen is part of the blood filtering process and is involved in both production and destruction of certain types of blood cells. Removal of the spleen is sometimes necessary for certain blood disorders involving red blood cells or platelets, intrinsic problems of the spleen, traumatic injury or if the spleen is enlarged from infective/inflammatory processes.
The spleen is part of the blood filtering process and is involved in both production and destruction of certain types of blood cells. Removal of the spleen is sometimes necessary for certain blood disorders involving red blood cells or platelets, intrinsic problems of the spleen, traumatic injury or if the spleen is enlarged from infective/inflammatory processes.