Long Covid Information
© North Bristol NHS Trust. This edition published August 2023. Review due August 2026. NBT003488
© North Bristol NHS Trust. This edition published August 2023. Review due August 2026. NBT003489
Resources for people who may be struggling with work because of Long Covid
These resources may help you and your employers if you are struggling with work because of Long Covid. We have chosen ones that contain a wide range of information for various challenges you may have. You might choose to share some of these with your employer.
Covid 19 return to work guide for managers
The "Covid 19 return to work guide for managers" by the Society for Occupational Medicine contains detailed advice on a phased return to work, which can be a very important for people with long Covid symptoms and may be a new concept for employers. It contains a comprehensive overview of work-related issues faced by employers and employees.
https://www.som.org.uk/COVID-19_return_to_work_guide_for_managers.pdf
C19 return to work guide for recovering workers
The “C19 return to work guide for recovering workers” (2021) by the Society for Occupational Medicine provides a comprehensive overview of managerial and employee duties and expectations.
https://www.som.org.uk/COVID-19_return_to_work_guide_for_recovering_workers.pdf
Webinars "Returning to work with on-going COVID-19 symptoms: Guidance for employees and managers
The webinars "Returning to work with on-going COVID-19 symptoms: Guidance for employees and managers" (2021) by University Hospitals of Derby and Burton NHS Foundation Trust provide information for managers and employees and covers practical steps.
https://www.youtube.com/playlist?list=PLfPD5ilcfEbit6R2OgezYD9YeNvnniSyb
"Long Covid FAQs" on the Chartered Institute of Personnel and Development website
The page "Long Covid FAQs" on the Chartered Institute of Personnel and Development website is directed at employers, and contains some useful facts and statistics, and some information on legal rights.
https://www.cipd.co.uk/knowledge/coronavirus/faqs/long-covid
Faculty of Occupational Medicine Guidance for managers & employers
The "Guidance for managers & employers on facilitating return to work of employees with post-COVID syndrome" (2021) by the Faculty of Occupational Medicine provides employers with general advice on returning to work, and some useful examples of reasonable adjustments.
https://www.fom.ac.uk/wp-content/uploads/FOM-Guidance-post-COVID_employers-managers.pdf
Access to Work
Access to Work can provide practical and financial support if you have a disability or long-term condition. This can be useful whether you have a job, are looking for work, are on supported internship or traineeship or want to become or are self-employed.
https://www.gov.uk/government/publications/access-to-work-factsheet
This video was delivered as a webinar and provides an overview of Long Covid and the impact on people’s working lives and presents key strategies and principles that can support people to return to work and to stay in work.
Contributors:
Dr Tim Robinson provides an overview of the symptoms and management of Long Covid. Dr Robinson works in the Community Long COVID Single Point of Access assessment clinic which covers Bristol, North Somerset and South Gloucestershire (known as BNSSG).
Fiona Mckechnie shares approaches and adjustments that have been used to support people with fatigue syndromes in the workplace. Fiona is an Occupational Therapist in the Bristol M.E. Service, North Bristol NHS Trust.
If you have Long Covid and would like to have support, please contact your GP to request a referral to your nearest Long Covid clinic.
Transient Ischemic Attack (TIA) Clinics
TIA is a medical emergency - anyone suffering a suspected TIA should see a doctor immediately.
You may be referred to the TIA clinic because you had symptoms suggesting you’ve had a transient ischaemic attack (TIA), or a minor stroke. A TIA is sometimes called a mini stroke.
Why is an urgent appointment with a stroke specialist so important?
About one in ten people who have a TIA develop a stroke within the next week, but we can greatly reduce this with the right treatment.
The right medication can reduce your chance of a stroke, as can changes to your diet and exercise. Rarely an operation may be useful. We will discuss all this when you see us in clinic.
What happens now?
The clinician who initially diagnosed you may give you medication to take. You need to take this each day until you are seen in our clinic. They will also make a referral to the TIA Service.
TIA clinics run daily, including weekends. Every day, the stroke specialist on duty will review all new referrals. If we decide that we need to see you urgently over the weekend or at a bank holiday, the co-ordinator of the TIA Service will phone you on the day tell you what time to come to the unit.
Otherwise, the TIA co-ordinator will be in touch on the next working day to arrange an appointment for you to come in. In some cases, the appointment will be a telephone consultation only. The appointment will usually be on the same day. If you have not heard from us within two working days, please ring the TIA co-ordinator on 0117 342 4800.
Where are the TIA clinics?
Across the Bristol, North Somerset and South Gloucestershire (BNSSG) region, TIA clinics are operated centrally by University Hospitals Bristol and Weston (UHBW) NHS Trust.
There are clinics at Bristol Royal Infirmary and Weston General Hospital and weekend clinics at Southmead Hospital. If you are told to attend a TIA clinic, the TIA co-ordinator will tell you which clinic to attend.
Bristol Royal Infirmary
On week days days, the TIA clinic co-ordinator will tell you where you need to go. This will generally be the TIA Outpatient clinic A403 (Zone A, Level 4). Sometimes they will ask you to go straight to the radiology department to have a brain scan (Location A217, Zone A, Level 2) or to the vascular sciences department for a carotid doppler scan (Location A225, Zone A, Level 2).
Weston General Hospital
On week days, the TIA clinic co-ordinator will tell you where you need to go. This will generally be the TIA Outpatient Clinic within The Main Outpatients area of Weston General Hospital. The Main Outpatients is located at the front of the hospital.
Southmead Hospital
At weekends, clinics will be delivered at Southmead Hospital by the stroke/neurology service (Location Gate 36).
What can I expect to happen at my appointment?
A stroke specialist clinician will ask you to tell them about the symptoms, your past medical history, and what medicines you are currently taking. This will be followed by a physical examination. The clinician will discuss your diagnosis with you. You may be given new medications or a prescription. If you currently pay for your prescriptions, please ensure that you have a means of payment with you
What may we need to do? (Each case is different – you may not need all these investigations)
-
- A blood pressure check
- A heart recording (ECG)
- A scan of the arteries in your neck
- A brain scan
- A blood test
- A consultation with a stroke specialist - this may be a consultant, registrar or an advanced nurse practitioner for stroke
You can expect to be at the clinic for at least 3-4 hours and the appointment may take most of the day
What do I need to bring?
Please bring a list of all the prescribed tablets you take – this is very important. If there are other tablets you buy over the counter, please tell us about these. Please also bring any medications you will require throughout the day. If possible, it will be helpful for the person who witnessed you having your symptoms to come to the appointment as well.
There are cafes and shops all locations where you can purchase refreshments. Alternatively, you may want to bring your own provisions.
What about driving?
It is not safe or legal for you to drive until you have been seen in clinic. We will discuss any possible further driving restrictions related to your diagnosis with you in the clinic.
What if I have further symptoms before the specialist appointment?
If you think you are having a stroke, don’t wait – call 999!
What do I need to do?
Please bear these points in mind:
-
- DO NOT DRIVE until you are assessed at the clinic
- it would be helpful if anyone who witnessed your symptoms could accompany you to the appointment
- bring any medications that you are taking, or your repeat prescription order form
- eat and drink as normal
- your appointment may take several hours.
- If you have any recurrence of symptoms before your clinic appointment and they resolve within 5 minutes please call NHS 111, if they do not resolve within 5 minutes call 999.
Contact TIA Clinic
Telephone: TIA Co-ordinator 0117 342 4800
Email: BNSSGTIAService@uhbw.nhs.uk
COVID-19 Discover Study
With ongoing support from Southmead Hospital Charity, the DISCOVER study aims to collect blood samples and other routinely collected microbiological samples as well as medical information from patients with suspected coronavirus.
These blood samples will be analysed for both routine and new tests. Patients will be followed up with after one month to see how coronavirus affected them, and to see how useful the blood tests were at predicting their condition.
Biological samples will also be stored (anonymously) for future research on how COVID-19 makes people ill. In the future, this information could help doctors decide which tests are useful in managing coronavirus.
Dr David Arnold, a Respiratory Doctor at NBT, shares more about the importance of the DISCOVER study:
Video Transcript
Hello, my name is David Arnold, I'm a Respiratory Doctor here at Southmead Hospital and part of the NBT COVID-19 Research Team.
The research response at NBT to COVID-19 has been amazing, with nearly every patient recruited to at least one of our open clinical studies. One of these studies is called the DISCOVER study which is NBT led and set up by me and one of the Microbiology Doctors, Fergus Hamilton.
The Southmead Hospital Charity is supporting us in continuing the DISCOVER study, which aims to analyse blood-based biomarkers from patients who are admitted with the coronavirus.
Because one of the really important decisions that healthcare professionals have to make when seeing a patient with COVID-19 is who can be safely discharged home to recover there and who needs to be admitted to hospital for closer monitoring and support.
But we can do so much more with these samples as well. And with collaborations with the University of Bristol's UNCOVER group, as well as Public Health England, we can look at immunity following the virus, as well as secondary infections and respiratory compromise when people are recovering.
Study Results:
Patient outcomes after hospitalisation with COVID-19 and implications for follow-up; results from a prospective UK cohort
Of those who participated in the DISCOVER study, researchers have found that three quarters of the group who received care for COVID-19 were still suffering ongoing symptoms three months later.
- 81 out of 110 discharged patients were still experiencing symptoms such as breathlessness, excessive fatigue and muscle aches when invited back to clinic.
- Many were also suffering from poor quality of life compared to the rest of the population, struggling to carry out daily tasks such as washing, dressing or going back to work.
Most of the patients did, however, report improvements in their initial symptoms of fever, cough and loss of sense of smell. Reassuringly, the majority of patients had no evidence of lung scarring or reductions in lung function.
Speaking of the findings, Helen Lewis-White (Deputy Director Research & Innovation) said:
“There’s still so much we don’t know about the long term effects of coronavirus, but this study has given us vital new insight into what challenges patients may face in their recovery and will help us prepare for those needs.
We’re pleased that researchers at Southmead Hospital are leading the way, and hope our findings can help patients and their GPs understand the course of post-COVID illness and the role of routine tests."
To find out more about these discoveries, read the full pre-print report.
Please note this is a preprint, so it is a preliminary piece of research that has not yet been through peer review and has not been published in a scientific journal – so this is early data.
Are vaccines safe in patients with Long COVID? A prospective observational study?
The DISCOVER study followed a cohort of patients who were admitted with COVID-19 in 2020, many of whom remain highly symptomatic. It then compared those who received a vaccine to those who didn’t.
- 94% of participants had no worsening of symptoms after receiving the vaccine compared to 86% of unvaccinated sufferers from the same study.
- 23% of the patients with Long COVID actually saw an improvement in symptoms after receiving a vaccine, compared with 15% of the same Long COVID patient group who were unvaccinated.
The study follows uncertainty and concern amongst Long COVID sufferers on the impact the vaccine may have on their symptoms, with anecdotal reports suggesting both worsening and improvement of symptoms.
The study has found that for patients suffering with Long COVID, receiving a COVID-19 vaccine did not worsen their symptoms, which will be of great reassurance to many.
Speaking of these findings, Dr Fergus Hamilton from North Bristol NHS Trust said:
“This is really positive news for those with Long COVID. This study confirms that the COVID-19 vaccines do not worsen symptoms with a hint that they could actually improve them for some people. We hope this provides reassurance for anyone with Long COVID who may have been hesitant to get the vaccine, and we would encourage everyone to get the jab when invited.”
To find out more about these discoveries, read the full pre-print report.
Please note this is a preprint, so it is a preliminary piece of research that has not yet been through peer review and has not been published in a scientific journal – so this is early data.
Thank you to all of our research teams who are making such a different to people’s lives, and also to Southmead Hospital Charity which is raising much-needed funds for COVID-19 research.
Take Part in Research

Become one of the thousands of people taking part in research every day within the NHS.
Contact Research
Research & Development
North Bristol NHS Trust
Level 3, Learning & Research building
Southmead Hospital
Westbury-on-Trym
Bristol, BS10 5NB
Telephone: 0117 4149330
Email: research@nbt.nhs.uk
Point of Care

Point of care testing (POCT), also known as extra-laboratory testing or Near Patient Testing (NPT), refers to any analytical test performed for or by a patient outside the conventional laboratory setting.
The Point of Care Testing Team provides support for many POCT applications to North Bristol NHS Trust.
The service encompasses:
- Advice on the selection and procurement of POCT equipment.
- Maintenance and servicing of blood gas analysers across the Trust.
- Replacement and servicing of blood glucose/ketone meters.
- Supply of consumables for blood glucose meters including quality control solutions, batteries and user manuals.
- Supply of Pregnancy testing kits (via Pharmacy).
- Training in blood gas analysis, blood glucose analysis, capillary blood collection, urinalysis, haemoglobin testing and pregnancy testing.
- Training materials for urinalysis and pregnancy testing.
- External Quality Assurance schemes for blood glucose and some other POCT tests.
- Support for Haematology analysers situated in Out Patients departments and Operating Theatres.
- Covid-19 testing using Abbott ID Now devices.
Please also see the results of our latest POCT User Survey.
Updated 26/10/2021
Point of Care Testing
The department is open 9am to 5pm Monday to Friday.
Pathology Sciences Laboratory,
Southmead Hospital,
Westbury-on-Trym,
Bristol
BS10 5NB
Email: POCT@nbt.nhs.uk
Telephone: 0117 4148422
POCT Information on LINK

All POCT documentation (including SOPs, Quickguides and manuals) can be found on the POCT pages on LINK.
COVID-19 CCP-Cancer Study
Currently, there is extremely limited information regarding the risks posed by SARS-CoV-2 virus responsible for COVID-19 to patients with cancer.
This study aims to understand the presentation, management and outcomes of cancer patients with COVID19. The influence of cancer type and treatment will be explored as well as comparing cancer patients with non-cancer patients.
This study will provide valuable information that would educate as well as help inform practice for future possible outbreaks. The information may also inform the development of guidelines with regard to the care and management of cancer patients with viruses such as COVID19 and similar infectious diseases.
Cancer is immunosuppressive, the nature of the immunosuppression seems to be influenced by the microbiota, in addition pulmonary infections are also influenced by the host microbiota, therefore it is important to understand this impact in cancer patients. Analysis of samples from cancer patients with COVID19 offer the opportunity to learn more of these interactions.
The purpose of CCP-CANCER UK study is to obtain additional data from patients with cancer who were/are recruited into the Principal CCP-UK study which is the key national protocol for characterising COVID19 in the UK population. This study is designed to supplement, not replace, the Principal CCP-UK protocol.
This study will be open to research sites who are currently participating in the Principal CCP-UK study.
The CCP-Cancer UK study will run for two years. An additional specific cancer data set will be collected from participant's existing medical records. This cancer specific information when combined with the rich data set related to the COVID-19 episode (derived from CCP-UK) will enable a full understanding of COVID-19 in patients with cancer as well as enable a comparison with non-cancer patients.
Take Part in Research

Become one of the thousands of people taking part in research every day within the NHS.
Contact Research
Research & Development
North Bristol NHS Trust
Level 3, Learning & Research building
Southmead Hospital
Westbury-on-Trym
Bristol, BS10 5NB
Telephone: 0117 4149330
Email: research@nbt.nhs.uk
Urology Research Team
The Urology Research Team at NBT is a multidisciplinary team of healthcare professionals, academics and members of the public working across both functional urology and oncology.
Our research is supported by the Department of Health and the Bristol Urological Institute. We conduct research across a variety of settings, including laboratory based work and clinical work.
We work on a range of commercial and non-commercial, qualitative and quantitative research and collaborate with colleagues locally, nationally and internationally in order to improve outcomes for patients suffering from various urological conditions, including but not limited to:
- Cancers such as prostate, bladder, kidney, penile and testicular
- Male and female incontinence
- Kidney disease including stone problems
- Uretic disease
- Benign prostate disease
We have close links with the University of Bristol and University of the West of England (UWE). We also work with patient groups such as Prospect.
Meet the team:
Becky Cousins - Lead Research Nurse
Lead Research Nurse
Becky currently works as the Lead Research Nurse for Urology at Southmead Hospital, managing a team of 6 highly competent research practitioners. Prior to her training, she completed her degree in Biomedical Sciences and Masters of Research at Cardiff University. Having qualified as an adult nurse from City University in 2013, she began working on a complex older adult ward at Whipps Cross Hospital, London. Whilst working in a clinical role she became focused on making an impact on quality of life through her job and keen to identify ways she could use her skills in research. After seeing an advert for the Huntington’s Disease (HD) research team at Cardiff University, she headed back to Wales in 2014 taking on a full-time role as a research nurse. She led on drug trials, longitudinal and academic studies, for which she gained a deep interest and passion. She joined North Bristol NHS Trust in 2019 as a research nurse with the ReMemBr group, focussing her passion for dementia research. Recently, she took a new path and joined the Urology Research team as the team lead, focusing on expanding the research portfolio and delivering a first in human catheter study. Becky has a keen interest in quality of life research, focussing on how we value people living in residential care. She is driven to see her team succeed; pursue their own research ideas and become highly qualified health-care professionals.
Marta Cobos-Arrivabene - Research Nurse
Research Nurse
Marta joined the Urology Research team in May 2021 and works primarily in functional urology. She qualified as a Registered Nurse in 2018 at the University of the West of England. Previously she worked as a recovery nurse in Medirooms at NBT where she covered different surgical specialities including urology. Her interest in research started during her student days when she had a placement with the research team. Since then, her passion about finding out about the latest treatment options for her patients and further understanding patient diagnoses and disease prevention has grown.
Victoria Garner - Research Nurse
Research Nurse
Victoria is the newest member of the Urology research team, having joined in June 2021. She has a degree in Medical Physiology from the University of Leicester, and in Adult Nursing from the University of Birmingham, and qualified as a nurse in 2014. Victoria has worked mostly in London, initially in Intensive Care at Kings College Hospital, before moving to Neonatal Intensive Care at St Georges Hospital. While this is the first role Victoria has had in research, it has been an area of great interest to her for many years, and she is passionate about pursuing a role in developing and improving the care available to people. Victoria works primarily in functional urology, on the PriMUS and PURSUIT trials.
Liz Goff - Research Administrator
Research Administrator
Liz joined the Urology Research team in April 2024 as a research administrator. Her role involves assisting the team with the daily administrative elements of running clinical research studies, helping with diary management, data entry and queries, document management and archiving, study finances and liaising with external sponsors.
Take Part in Research

Become one of the thousands of people taking part in research every day within the NHS.
About Research & Development

Find out more about our research and how we're working to improve patient care.
Contact Research
Research & Development
North Bristol NHS Trust
Level 3, Learning & Research building
Southmead Hospital
Westbury-on-Trym
Bristol, BS10 5NB
Telephone: 0117 4149330
Email: research@nbt.nhs.uk
Nutrition & Dietetics Nutrition Support
Malnutrition is a serious condition that happens when your diet does not contain the right amount of nutrients. Signs of malnutrition include weight loss, increased tiredness and poor wound healing.
If you or someone you know is at risk of malnutrition it is important to recognise and treat early.
Calculate the risk of malnutrition for you or someone you know.
There are many causes of malnutrition including loss of appetite, difficulty swallowing and changes to taste and smell. If left untreated, malnutrition can cause loss of muscle strength, increased risk of infection and low mood.
If you are in the community and are worried that you or someone you know is at risk of malnutrition, take a look at the following resources. If weight loss continues or you have already lost a lot of weight without meaning to, please speak with your GP and they may refer you to a community dietitian.
For ways to increase energy and protein intake at home, click on the below links:
- High Protein Foods
- The importance of protein
- Increasing Energy and Protein intake
- Malnutrition: Food Fact Sheet
For ways to increase variety in your diet, click on the below links:
- Fruit and vegetables – how to get five-a-day
- Information on Iron
- Information on Vitamin D
- Information on Fibre
- Do I need supplements?
- Eat Well, Spend Less
If you or someone you know is in hospital take a look at the following resources.
For ways to increase energy and protein intake in hospital:
Additional information
For information on nutrition and COVID 19: Malnutrition Pathway COVID-19 Leaflets
For information on malnutrition and specific conditions: www.malnutritionpathway.co.uk/specific-resources
For information on diet and specific conditions:
www.bda.uk.com/food-health/food-facts.html
www.bda.uk.com/resource/pressure-ulcers-pressure-sores-diet.html
Contact Nutrition & Dietetics
Kendon House
Kendon Way
Southmead Hospital
Bristol
Telephone: 0117 414 5428 or 0117 414 5429
FIT Testing for Patients
Information for Patients
Faecal Immunochemical Test (qFIT) for Occult Blood in Faecal Samples
What is being tested?
The faecal immunochemical test (qFIT) checks for blood in your faeces. Normally, only a very small amount of blood is lost in the stomach or intestines. This is less than you can see in your faeces and is not enough to be detected with a FIT test.
A positive FIT test will tell your doctor that you have bleeding occurring somewhere in your gastrointestinal tract. This blood loss could be due to ulcers, bulges, polyps, inflammatory bowel disease, haemorrhoids (piles), swallowed blood from bleeding gums or nosebleeds, or it could be due to early bowel cancer. Anything that sticks out into the intestine, like a polyp or tumour, and is rubbed against by the faeces as it passes through, has the potential to bleed now and again. Often this small amount of blood is the first, and sometimes the only, symptom of early bowel cancer.
Which patients are eligible for FIT?
The main use for the FIT test is as to find early bowel cancer. Blood in the stool may be the only symptom of early cancer. If the cancer is detected before it spreads to other areas, there is a greater chance it will be cured. The signs and symptoms of bowel cancer are not always easy to see. In some patients, with an abdominal or rectal mass, rectal bleeding, anal ulceration or if they are age >60 y with iron deficiency anaemia. Your GP will request you are seen by the hospital urgently as a “two week wait patient” and you will not be offered a FIT test. In other patients where the signs and symptoms are less clear your GP may think you could have bowel cancer but wants to be more certain that this is the case. In these circumstances a FIT test will help them decide.
You also need to meet some other criteria to be offered the test; these criteria have been developed by the National Institute for Clinical Excellence and local cancer services after reviewing all the evidence.
- • Weight loss, abdominal pain, change in bowel habit (age >40 years)
• Iron deficiency anaemia (age <60 years) or a non-iron deficiency anaemia (age >60 years)
• Change in bowel habit or other symptoms that could be caused by colorectal cancer but are low risk (age >18 years)
How do you get tested?
Your GP will give you a pack containing all the information and equipment you need to collect your faecal sample and send it directly to the laboratory or return it to the GP practice to forward to the laboratory


Inside the pack you will find the
• Collection device
• Request form (for return to GP pack this will be generated by the practice)
• Instruction sheet
• Return envelope


Your GP should have completed the request form, if this has not been done then please ask the practice to do this for you.

Read the instructions carefully
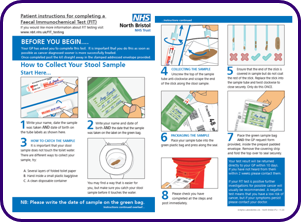

Click here to see a full sized version of the instructions:
When you have collected your sample

How soon can I expect the results?
GP’s will receive the results of the analysis within 7 working days from the date the test is sent to the laboratory. Your GP may then need to decide what is the next best step for you but if you have not heard within 10-14 days please contact the practice.
What do the results mean?
If the Faecal occult blood test is negative: If your result is negative (Faecal occult blood (FIT) <10 μg Hb/g faeces) it is very unlikely you have bowel cancer. You therefore do not need referral for suspected bowel cancer. If your GP is concerned that worrying symptoms persist they may do further tests or refer you for specialist advice. This will be done in line with local arrangements.
If the Faecal occult blood test is positive: If your result is positive (Faecal occult blood (FIT) >10 μg Hb/g faeces), this does not necessarily 'prove' that you have cancer. There are other possible causes of bleeding and other follow-up procedures will need to be done to find the source of the bleeding. Your GP is likely to refer you for further investigations and this may be done urgently
Where can I access further information and support?
• For more information about FIT the following website might be of interest: www.faecal-immunochemical-test.co.uk/
• For more general information about laboratory tests: https://labtestsonline.org.uk/

To contact the laboratory who are going to process your sample?
Please email the laboratory on nbn-tr.nbtfit@nhs.net
Page updated 28/09/21
Test Information

Includes details of sample types, volumes, special precautions, turnaround times & reference ranges.
