Consumables Ordering
Pathology Consumables are ordered using the pathology consumables order form below.
If you have any questions about your order please email pathologyconsumablessouthmead@nbt.nhs.uk
Pathology Consumables are ordered using the pathology consumables order form below.
If you have any questions about your order please email pathologyconsumablessouthmead@nbt.nhs.uk
Advance Care Planning (ACP) is a process of often several voluntary discussions between an individual and any health care professional who knows them. Aspects of ACP may include:
If the individual wishes, their family and friends may be included in the discussions. With the individual's agreement, this discussion should be recorded, regularly reviewed and communicated to key persons involved in their care.
Not everyone wishes to have conversations about these issues and there should be no obligation to do so.
Advance Statement (also known as Preferred Priorities for Care (PPC))
An advance statement is a written statement that allows a persons preferences, wishes, beliefs and values regarding their future care to be formally documented. The aim is to provide a guide to anyone who might have to make decisions in the persons best interest if they have lost the capacity to make decisions or to communicate them. An advance statement is not legally binding, but anyone who is making decisions about an individuals care should take it into account.
An Advance Decision to Refuse Treatment (ADRT)
An Advance Decision to Refuse Treatment (previously called a Living Will or Advance Directive) is different from a Statement of Wishes and Care Preferences as it is a formal, legally binding document or verbal statement which allows the refusal of certain medical treatments by an individual who is over 18 and has capacity to make the decision. It cannot be used to demand medical treatments which are not thought to be of benefit to the individual, request to have life ended or the refusal of basic essential care. An Advance Decision to Refuse Treatment which includes reference to life sustaining treatment must be in writing. A compliant advance decision is as valid as a contemporaneous decision, that is, one made at that time. An Advance Decision to Refuse Treatment must exist, be valid and be applicable to the current circumstances.
Lasting Power of Attorney
A Lasting Power of Attorney is a legal document. It allows the individual (known as the donor) who has capacity, to appoint someone they trust as an ‘attorney’ to make decisions on their behalf. The circumstances under which attorneys can make decisions depends on the type of LPA.
There are two types of Lasting Power of Attorney.
1. Health and Welfare/ Personal Welfare Lasting Power of Attorney
This type of LPA may give the individual’s attorney(s) the right to make decisions on behalf of the individual e.g.
This type of LPA only comes into force if the individual loses the ability to make decisions and is only valid once it has been registered with the Office of the Public Guardian.
2. Property and Affairs Lasting Power of Attorney
This type of LPA may give the individual’s attorney(s) the ability to make financial decisions for them e.g.
The individuals attorney has the authority to take over the management of their financial affairs as soon as the LPA is registered with the Office of the Public Guardian, unless the LPA states that this can only happen if they lose mental capacity to manage their own affairs.
There are special rules about appointing a LPA. Please visit the website Office of the Public Guardian at www.gov.uk/office-of-public-guardian telephone 0845 330 2900.
Further information about Advance Care Planning
Planning Your Future Care - A Guide - This booklet provides an explanation about advance care planning and the different options available, visit www.nhs.uk/Planners/end-of-life-care/Pages/planning-ahead/advance-statement
Advance Statement (also known as Preferred Priorities for Care) - For further information and examples of documents to help write down preferences and wishes for the future, visit www.nhs.uk/Planners/end-of-life-care/Pages/advance-statement and www.westonhospicecaregroup.org.uk
Office of the Public Guardian - To help with protection of people who lack capacity, visit www.publicguardian.gov.uk
Making Decisions- A Guide - Information booklets about the Mental Capacity Act, visit www.dca.gov.uk/legal-policy/mental-capacity/mibooklets/booklet01.pdf
Advance Decision to Refuse Treatment - For further information visit www.nhs.uk/Planners/end-of-life-care/Pages/advance-decision-to-refuse-treatment and www.macmillan.org.uk/Cancerinformation/Livingwithandaftercancer/Advancedcancer/AdvanceDecision
Gold Standards Framework - Is an approach to optimising the care for patients nearing the end of life delivered by community health care teams. It is concerned with helping people to live well until the end of life and includes care in the final years of life for people with any end stage illness in any setting. visit www.goldstandardsframework.org.uk/patients-amp-carers
You may be able to get help to access these through your Community Nurse, GP or Hospital Specialist Nurse.
Reviewed on 20/10/21
The Screening Tests for You and Your Baby leaflet contains information about the blood spot screening test and the conditions it screens for. It is available in other languages.
The NHS Newborn Blood Spot Test website includes information about each condition, FAQ's and links to further information.
Screening is less straightforward if your baby has had a transfusion or is born at less than 32 weeks of pregnancy, an information leaflet can be found at www.gov.uk/government/publications/screening-tests-for-you-and-your-baby-babies-in-special-care-units
Details about how cards are stored, and who can access them can be found here.
NHS Trusts are required by law to make Standing Orders (SOs), which regulate the way in which the proceedings and business of the Trust will be conducted.
High standards of corporate and personal conduct are essential in the NHS. These “extended” Standing Orders, incorporating the Standing Financial Instructions (SFIs) and Scheme of Delegated Authorities (SoDA) identify who in the Trust is authorised to do what.
Download:
Links to guideline and information resources
American Society of Hematology
British Society of Blood and Marrow Transplantation
British Society of Haematology
European Hematology Association
National Institute for Health and Clinical Excellence
National Cancer Research Institute
Sources of information for patients
Lymphoma Association
This statement outlines the purpose and functions of the Haemato-Oncology Diagnostic Service at North Bristol NHS Trust.
Internal quality assurance
Standard operating procedures are used for diagnostic investigation and consistent reporting. BHODS uses the following approaches to maintain quality of sample processing and results reporting.
A SOP dealing with for registration of patient details, clinical information and recording of samples received.
A SOP by tissue type for initial and subsequent investigation panels.
A SOP for reporting and authorisation.
A SOP based on the WHO 2017 classification gives the diagnostic standards for haematological malignancies. It identifies the disease to be diagnosed, ICD / SNOMED code, relevant prognostic factors and any differences from the WHO criteria in making the diagnosis if appropriate.
Interim reports may be issued when the diagnosis is made but additional information such as that required for prognosis is pending Interim reports will be finalised with a “Final report” and any corrections to a final report will be amended with an “addendum” report.
A typical, unusual or rare cases will be independently checked before authorisation and report issue.
SOP's are reviewed annually and are in line with those of other similar diagnostic services.
Trusts sending samples to BHODS are offered regular input for clinical MDT meetings Laboratory IT systems are used to check reporting accuracy, reporting concordance and reporting times.
The process of multidisciplinary investigation of Haematological Malignancy will result in a small number of cases where either diagnostic modalities or reporting clinicians are unable to define a single unifying diagnosis. These cases are subject to multi-disciplinary review within NBT SIHMDS Diagnostic Review Meeting.
The service has target turnaround times for investigative processes. The majority of cases will be dealt within the target times but in some situations it may prove impossible to meet these by reason of technical or complicated diagnostic pathways.
Bristol Haemato-Oncology Diagnostic Service Reporting Times
| Tissue | Target Reporting Time3 |
|---|---|
| Bone Marrow1 | 7 days |
| Tissue2 | 10 days |
1. Includes haematological morphology, immunophenotyping and trephine histopathology
2. Includes lymph node morphology and immunohistochemistry
3. Where supplementary tests are required the target reporting times are as detailed below
Haematological morphology
| Investigation | Target Reporting Time |
|---|---|
| Peripheral blood morphology | 24 hours (working week) |
| Bone marrow morphology | 72 hours (working week) |
Immunophenotyping
| Investigation Target | Target Reporting Time |
|---|---|
| Haematological malignancies - urgent | within 4 hours of receipt |
| Haematological malignancies - routine | 1 working day |
| PNH Screen | 1 working day |
Histopathology
| Tissue/process | Target Reporting Time |
|---|---|
| Lymph node morphology | 3 working days |
| Lymph node immunohistochemistry | 4 working days |
| Bone marrow trephine | 4 working days |
| Bone marrow immunohistochemistry | 7 working days |
Cytogenetics
| Investigation | Target Reporting Time |
|---|---|
| Urgent karyotype e.g. AML, ALL, CML | 7 calendar days |
| Routine karyotype e.g. MDS, MPN | 21 calendar days |
| Urgent (Priority 1) FISH e.g. BCR/ABL1, PML/RARA | 3 working days |
| Urgent (Priority 2) FISH e.g. CLL, FFPE | 14 calendar days |
| Routine (Priority 3) FISH e.g. Myeloma | 21 calendar days |
Molecular Pathology
| Investigation | Target Reporting Time |
|---|---|
| Quantitative BCR-ABL1 | 3 working days |
| Quantitative BCR-ABL1 monitoring in CML and ALL | 14 calendar days |
| ABL1 kinase domain mutation testing | 28 working days |
| JAK2 (V617F) mutation analysis | 14 calendar days |
| CALR (exon 9) mutation analysis | 14 calendar days |
| JAK2 (exon 12) mutation analysis | 28 working days |
| MPL (exon 10) mutation analysis | 28 working days |
| KIT (exon 8 and 17) mutation analysis | 14 calendar days |
| IG/TCR clonality assessment | 14 calendar days |
| IGVH mutation and gene usage in ALL | 14 calendar days |
| BRAF (V600E) mutation testing | 14 calendar days |
 Investigative techniques: Morphology/Cytology
Investigative techniques: Morphology/Cytology
Consideration of the clinical information, blood count results and conventional light microscope morphology of marrow or blood helps to determine the most appropriate panel of investigations form a number of techniques.
Investigative techniques: Flow Cytometry
Provides a method of rapidly enumerating and characterising the antigen expression of cells in suspension using a panel of antibodies labelled with florochromes.
In the haematopathlogy field the technology can be used to distinguish chronic lymphoproliferative disorders and separate these from reactive states; define acute leukamias, identify prognostic markers and monitor minimal resisdual disease; count specific cells types and identify condition associated with aberrant antigen expression.
Investigative techniques: Histopathology
The assessment of fixed tissue prepared as tissue sections is used for the examination of lymph nodes, marrow trephines as well as potential tumours from any body site. Histopathology of lymph nodes is the best method to diagnose and classify lymphoma. Fresh node sent to the laboratory allows the use of flow cytometry to facilitate the diagnosis. Frozen, fixed or paraffin embedded can be assessed by morphology and immunohistochemistry. If appropriate clonality of lymphoid populations can be assessed by IgH and TCR gene rearrangement or FISH on paraffin embedded tissue can identify characteristic chromosome translocations.
Investigative techniques: Cytogenetics
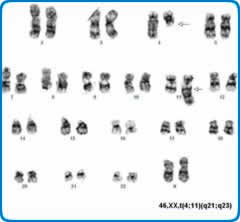
Conventional cytogenetics requires the culture of cells to provide metaphases. For this reason it takes longer to produce results than some other technologies but is a well-established technique able to provide results that are critical in the classification and prognostication of haematological disorders.
Investigative techniques: Fluorescence in-situ hybridisation (FISH)
FISH provides a method to identify changes that help define or provide prognostic information for lymphoid and myeloid disorders. The ability to use metaphase or interphase preparations is powerful and results are confirmed by subsequent conventional assessment whenever possible.
Investigative techniques: Molecular Genetics
The use of sensitive molecular techniques to assess DNA and RNA provide important and evolving tools. A range of techniques may be used for identifying mutations or molecular markers for diagnosis, prognosis and monitoring response to therapy and remission status. Examples of technologies used are qualitative and quantitative polymerase chain reaction assays, fragment analysis, methylation assays, and direct sequencing. Increasingly molecular ghenetic tests are moving across to next generation sequencing (NGS) technologies which allow the parallel investigation of multiple genes at increased sensitivity and resolution.
Investigative techniques: Whole Genome Analysis (WGA)
Following on from the 100K Genomes Project patients with acute leukaemia or any childhood patient with an Haematological cancer will be able to access WGA. WGA is an incredibly powerful technique which uses NGS to enable the simultaneous investigation of molecular genetic, FISH and cytogenetic diagnostic targets in a single, high resolution, assay. WGA requires simultaneous assessment of tumour (somatic) and normal (germline) material and therefore provides the additional benefit of identifying germline changes which may be contributing to the patient’s clinical presentation or have implications for treatment and management.
Results that fall outside the ranges below will be telephoned to the clinical area indicated on the request details except when they are not significantly different from previous values for a current admission.
Haematology
| Parameter | Telephone Ranges |
|---|---|
| White Cell Count | > 100.0 x 10*9/L |
| Neutrophils | < 0.5 or > 50.0 x 10*9/L |
| Haemoglobin | < 70 or > 190 g/L |
| Platelets | < 30 or > 1000 x 10*9/L |
| Plasma Viscosity | > 5.0 mPa |
| Malaria Parasite | Always telephone a positive result |
| Sickle Screen | Urgent positive results |
Coagulation Studies
| Parameter | Telephone Ranges |
|---|---|
| Fibrinogen | < 0.5 g/L |
| Warfarin Monitoring | INR > 6.5 |
| Heparin Monitoring | APTT > 135 secs |
Blood Transfusion
| Parameter | Telephone Ranges |
|---|---|
| Neonatal DAT | Always telephone a positive result |
| Positive Antibody screen | Positive antibody screens will be phoned to the clinical area if it will impact on the provision of red cells. |
| Foetal Leak | >4 ml bleed
|
Errors
| Wrong Blood In Tube | These will be communicated to the clinical area. Repeat samples are required. |
|---|---|
| Rejected Crossmatch Requests | The lab will make reasonable attempts to make contact with the clinical area to request a repeat. |