What is the placenta?
- The placenta (afterbirth) is an organ that develops along with the baby inside your uterus (womb) during pregnancy.
- It attaches to the wall of your uterus and provides a connection between you and your baby.
- Oxygen and nutrients pass from your blood through the placenta into your baby’s blood.
- The placenta is often called the afterbirth because it will normally be delivered shortly after the baby is born.
- Rarely, pregnancy may be complicated by a problem known as placenta accreta spectrum (PAS).
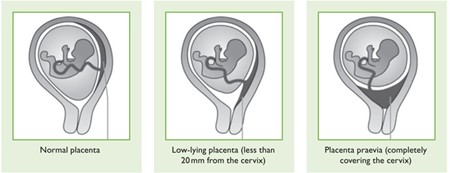
What is placenta praevia?
In some cases, the placenta attaches low down in the uterus and may cover part of or all of the cervix (the neck of the womb). In most cases, the placenta moves upwards and out of the way as the uterus grows during pregnancy. For some people, the placenta continues to lie in the lower part of the uterus as the pregnancy continues. This condition is known as low-lying placenta. If the placenta completely covers the cervix this is known as placenta praevia. If the placenta is very close (<20mm) or blocking the birth canal, you will need to have a caesarean birth.
What is Placenta Accreta Spectrum (PAS)?
- PAS is a rare complication of pregnancy (between 1 in 300 and 1 in 2,000 pregnancies).
- PAS covers a range of clinical conditions where the placenta is abnormally attached into the muscle of the uterus, making separation at the time of birth difficult.
- PAS is more commonly found in women with placenta praevia and who have previously had a caesarean birth.
- It can also occur if you have had other surgery to your uterus, such as removal of a fibroid (myomectomy) or uterine curettage.
- It is more common if you are older (over 35 years old) or if you have had fertility treatment, especially in vitro fertilisation (IVF)
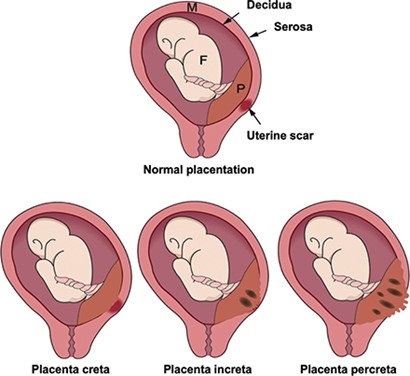
What are the different types of PAS?
There are three main types of PAS defined according to the depth of placental invasion into the muscle of the uterus (myometrium). They have been classified by the International Federation of Gynaecology and Obstetrics (FIGO):
- Placenta accreta (FIGO 1): the placenta firmly attaches to the superficial layers of the myometrium.
- Placenta increta (FIGO 2): the placenta is more deeply embedded into the myometrium.
- Placenta percreta (FIGO 3): the placenta passes through the myometrium and attaches to the outer layer of the uterus (serosa), and sometimes becomes attached to adjacent organs such as the bladder.
Diagnosis of PAS
PAS can be seen on ultrasound imaging in approximately half of all cases, and in up to 80 - 90% of the time when ultrasound is done by PAS experts. Magnetic Resonance Imaging (MRI) may also be recommended in special cases. This does not involve radiation and is safe in pregnancy.
Place of care - The South West PAS Network
Evidence shows that women have fewer complications when PAS is identified before birth and when cared for by an experienced team from diagnosis to delivery. The South West PAS Network is located in Southmead hospital, North Bristol NHS Trust. It coordinates, provides and standardises care for pregnant women with potential or confirmed FIGO 2 and 3 PAS disorder. The overriding aim of the network is to ensure equitable access, excellent experiences and the best possible outcomes for women and their families from all communities across the South West.
What should you expect from us?
Antenatal care
You may not experience any bleeding during the antenatal period and can be managed at home.
Some do experience bleeding during the antenatal period. If this is heavy it may mean we need to deliver your baby early.
If this is not heavy, but repeated, it may be necessary for you to be admitted to hospital for observation because of the risk of sudden heavy bleeding. This could be a prolonged hospital admission for observation.
Once a decision has been made about timing of delivery, or should you be admitted with bleeding, you will be advised what to expect in terms of your baby’s prematurity, especially if a long stay in the Neonatal Unit is predicted.
During your pregnancy you will be given the opportunity to have contact with our specialist midwife who supports our patients in the PAS service, as this can be a very stressful time. They will also be in contact with you after the birth of your baby to offer further support.
Delivery planning
You will be seen by a specialist consultant obstetrician and anaesthetist to plan the birth. Delivery is often planned earlier - between 34 to 36 weeks, to reduce the risk of heavy bleeding, should labour begin. The exact time of delivery depends upon your individual circumstances. Recovery time after delivery depends upon the type and extent of treatment you have for PAS. If PAS is suspected before your baby is born, your doctor will discuss your options and the extra care that you will need.
Delivery
If you have PAS there is likely to be bleeding when an attempt is made to deliver your placenta after your baby has been born. The bleeding can be heavy and you may require a hysterectomy to stop the bleeding.
There is a risk of injury to your bladder during the delivery of your placenta, this depends on your individual circumstances.
Your team may discuss with you the option of a planned caesarean hysterectomy (removal of your uterus with the placenta still in place, straight after your baby is born) if PAS is confirmed at the time.
It may be possible to leave the placenta in place after birth, to allow it to absorb over several weeks or months. This type of treatment is often not successful and can be associated with very serious complications such as bleeding and infection. Some people will still go on to need a hysterectomy.
Your healthcare team will discuss a specific plan of care with you depending on your individual situation.
We may plan for other specialists to be present for your delivery, to manage your risk of heavy bleeding (such as an interventional radiologist or a vascular surgeon), and to deal with any complications that may arise from where the placenta is located (such as a specialist bladder or bowel surgeon).
Your Birth Plan
PAS can cause life-threatening bleeding at the time of birth, so we plan your care to manage this.
You will be seen by a consultant anaesthetist who will discuss the options available to you for anaesthesia and pain relief after the surgery.
We will discuss the need for blood transfusion and other treatments to replace the blood you may lose during the surgery.
This may also affect how your blood clots so treatments may be needed to correct this.
After surgery you may need admission to a Critical Care Unit to help your recovery, either on the labour ward or in the main Brunel building at Southmead hospital. You will also have involvement from specialist midwives and neonatology (baby doctors).
What should you do now?
Should you have bleeding, signs of labour such as contractions or the baby’s waters breaking, you should seek immediate medical assistance at your closest maternity unit.