This page is for patients considering sacral neuromodulation as a treatment to improve bladder function.
Introduction
Sacral neuromodulation is used to help treat certain bladder and/or bowel conditions. This leaflet aims to give you information about the procedure and how to contact us if you have any further questions.
Please read this leaflet carefully as it is important you understand what to expect. If you have any queries or concerns, there is a section at the back for you to write these down and ask the clinician during your next appointment.
What is sacral neuromodulation (SNM)?
SNM is an implanted system (a medical device) which can help improve bladder function by sending electrical signals to the nerves that control your bladder and pelvic floor. The device is similar to a pacemaker (used for the heart) and it can help restore normal function of your bladder.
The device is inserted into the lower part of your back (spine) and is made up of a wire and a battery. The battery is placed in your buttock.
What conditions are SNM used for?
SNM is used to treat the following urological conditions:
- Overactive bladder syndrome / detrusor overactivity - this is when you have a strong need to get to the toilet quickly (urgency), increased daytime frequency (needing to pass urine often), nocturia (passing urine at night), and/or being unable to get to the toilet in time and leaking urine (urgency incontinence) before you get to the toilet.
- Non-obstructive urinary retention / voiding dysfunction this is the inability to empty the bladder either fully or partially, with no physical obstruction to the urine flow.
The device does not work for everyone, which is why all patients must undergo a test phase to see if it is likely to work. We hope the device will significantly improve your symptoms, but it may not completely cure them. Our success rate is approximately 65-70%.
What are the alternative treatment options?
- Overactive bladder - you should have tried all conservative and drug treatments before SNM. Other treatment options include botulinum toxin injections (into the bladder) and more invasive surgical options.
- Urinary retention – catheters can be used to empty your bladder. Other options include a Mitrofanoff procedure (Bladder Procedures).
Who is suitable for SNM?
If other treatments have not worked then you may be suitable for SNM. Not all patients are suitable for this type of treatment and if this is the case for you, it will be discussed fully at your SNM appointment.
There are 2 stages to SNM:
- The basic or advanced evaluation test phases.
- The full system implant phase (permanent implant).
There are currently two SNM companies that provide these devices; Medtronic and Axonics. Both companies offer rechargeable devices, and Medtronic also supply a recharge-free (non-rechargeable) device. Axonics’ non-rechargeable device is undergoing regulatory approval and will be available in the future.
Generally, both procedures are done as a day case. The basic test phase is done under local anaesthetic in the urodynamics suite. The advanced test phase and permanent implant are done in the operating room (theatre) so you will be seen in the pre-operative assessment clinic by the anaesthetic team before this.
Throughout this process, you will be seen by a small dedicated team with specialist knowledge:
- Professor Hashim Hashim: Consultant Urological Surgeon responsible for the SNM service.
- Dr Laura Thomas: Consultant Clinical Scientist and contact for queries or concerns.
- Ms Anna Hassine: Urodynamic Technician and contact for queries or concerns.
Basic evaluation phase (test phase)
You will receive your appointment letter and paperwork prior to attending the appointment. Please contact us if you are currently taking any blood thinning medications, have any allergies, or could possibly be pregnant.
If your appointment is in the urodynamic suite then you can expect to be with us for about 2 hours, and can leave straight after your procedure.
The test stimulator kit

The test stimulator has 3 parts:
- An external white verifier which is connected to you via a white cable and this delivers the stimulation.
- Samsung handheld controller which is a touch screen and used to alter the intensity of the stimulation or to turn the device on/off.
- Thin wire which is inserted into the bottom of your back/ spine in the sacrum.
Before the procedure
- Your appointment is booked over the phone and paperwork sent out, and brought back on the day of the procedure.
- Pre-operative assessment is done if the procedure is being performed in theatre.
During the procedure
- You will be reviewed by the SNM team to gain consent and collect paperwork (3-day bladder diary, symptom and quality of life questionnaires) from you.
- Undress fully (in private) and change into a hospital gown.
- Lie on your front (face down) on the couch and we will clean your lower back with a cleaning solution (iodine or chlorhexidine).You will stay lying on your front for around 30-60 minutes.
- We will use X-ray to mark/draw, the location of the lead insertion and local anaesthetic will be injected.
- The lead is inserted down a thin needle placed in your lower back.
- Sticky dressings will be used to hold the lead in place and it will be connected to the stimulator box.
- You will go home with a controller (Samsung handset in image) which allows you to turn your stimulator on and off.
The device must stay in place for the whole test phase. Unfortunately, if the wire comes out there is no way to re-insert it without performing the whole procedure again. You therefore need to keep the dressing clean and dry. If it begins to peel off you can add additional dressings.
Please do not remove the dressings as that will pull the wire out.
Immediately after the procedure
- Your temporary implant will be switched on.
- You will be taught how to use the controller so that you can get the most improvement in your symptoms and at a level that is comfortable for you.
- When the implant is switched on, you will feel a tapping, pulling, or tingling sensation in the genital (vagina/scrotum) or rectal (anus) area. It should never be painful and the sensations are very mild. People tend to notice these sensations less over time as the body adjusts to the implant.
- If possible, you should bring a friend/relative to drive you home. If this is not possible please let a member of the team know and we will make sure you are feeling well enough to drive yourself home following the procedure. You will need to switch the device off while driving.
Are there any risks or side effects?
Most procedures have potential side effects. However there are very few risks associated with the test phase.
The most common side effects are discomfort around the insertion site and pain/tingling in your leg or foot. If this happens, we advise you to turn the level of stimulation down or switch the device off.
Please inform your SNM team if you experience any side effects so they can decide whether any further treatment is needed. Contact details can be found on the back of the leaflet.
What should I do when I’m at home?
The test period is usually between 1-3 weeks. During this time, please continue your normal activities but avoid physical tasks such as bending, stretching, or lifting heavy objects.
Do not get the dressings wet.
Please complete your bladder diary, quality of life and symptom questionnaires. It is vital you complete this paperwork. If you do not, we cannot consider you for a permanent implant.
When you leave the hospital, you will be given a discharge summary which contains important information about your temporary implant. If you need to call your GP or practice for any reason or to attend another hospital, please take this summary with you to allow the doctors to see details of your treatment.
What happens next?
You will have a follow-up appointment to return to the hospital, usually within 1-3 weeks. The information you give us at this appointment is very important, as it will help us decide whether you are suitable for the full system implant.
We will check how much your symptoms have improved during the test phase (this will be done by the whole SNM team collectively). At this stage, the temporary wire is removed in clinic and if the test phase has been successful, you will likely be offered a permanent implant. You will also need to return the handheld Samsung controller to us.
Frequently asked questions about the test phase
Can I work?
Yes you can, except if your work requires intense physical activities. If your job involves driving you can still do so but will need to switch your device off to drive.
Can I use my mobile phone?
Yes you can, there is no problem using any type of phone.
Can I shower or take a bath?
No – please avoid showering or bathing during the test phase. You need to avoid getting the dressing wet but can wash around this using a wet towel/wash cloth. It is important to keep the area around the dressings on the back dry.
Do I need to have my dressing changed?
No. The wire is temporary and held in place by the dressing. There are no stitches to hold it in place so you must not take the dressing off or allow anyone else to take it off.
Can I do sports activities?
You should restrict your physical activities due to the risk of the wire/lead moving from its position during the test phase.
Do I have to fill in a bladder diary?
Yes, you must fill it in during the test period. Without the information provided by the diary, it is not possible to properly assess whether you are suitable for a permanent implant.
Can I have sex?
We recommend that you avoid sexual intercourse (sex) during the test phase in case the wire/lead moves from the correct position.
Is the test reversible?
Yes, the test stimulation is reversible and can be stopped at any time.
Will you tell my GP?
Yes, we will keep them up to date with your progress and whether this treatment is suitable for you.
What is the usual level of stimulation?
There is no set level of stimulation that gives the best results. Every patient is different. Please leave the stimulation at a level which is comfortable for you and so you can just feel the sensation. There is no benefit to having it on a high level – it is not an endurance test!
Advanced evaluation (tined lead test)
In some cases where you have not had a completely successful test phase, maybe because your wire moved out of position early in the trial, your team may decide you would benefit from an advanced evaluation with a tined lead.
An advanced evaluation involves having the permanent tined lead/wire inserted in theatre, but once again connected up to an external battery pack as in the basic evaluation (test) section.
The benefit of this is that the permanent wire has small fixation points on it, called tines, which make it less likely to move out of position during the trial phase.
The disadvantage of an advanced evaluation is that the wire needs to be put in, in the operating room/theatre and you will then require a second surgical appointment 2-4 weeks after the insertion to either remove the wire if it has not worked or attach the neurostimulator (battery) to the wire if you have had a significant improvement in symptoms.
The process of lead insertion and following neurostimulator attachment are outlined in the full system implant section next.
Full system implant (permanent implant)
Implant phase
Three full-system implants are currently offered in the UK, all of which are safe to go in an MRI scanner.
- Medtronic InterStim X (recharge-free/non-rechargeable).
- Medtronic InterStim Micro (rechargable).
- Axonics Sacral Neuromodulation System (rechargeable).
Your clinician will talk you through each option and make sure you are happy with your choice. Once you have made an informed decision you will be placed on the waiting list for that particular device. Once this has been confirmed, you will receive another appointment for your pre-operative assessment before your admission date.
Medtronic InterStim™

Both Medtronic devices use the same handheld Samsung programmer and communicator. This enables you to adjust the level of the stimulation and allows you to turn your implant on or off.
The difference between the Medtronic devices are the implanted batteries and they are explained on pages 14 and 15.
What is an InterStim™ X SureScan™?
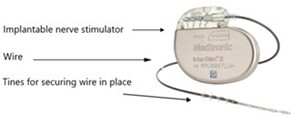
The InterStim X System consists of:
- An implantable pulse generator (IPG/battery) which is inserted under the skin. Usually in the buttock area.
- An electrode or thin wire with barbs/tines that carries the electrical pulses to the bladder nerves.
This full system implant does need recharging and has an estimated battery life of 12 years before the battery will need replacing. The lead stays in its position and is not changed.
What is an InterStim™Micro™SureScan™?


The InterStim Micro System consists of:
- An implantable pulse generator (IPG/battery) which is inserted under the skin (size of a small USB stick). Usually in the buttock area.
- An electrode or thin wire with barbs/tines that carries the electrical pulses to the bladder nerves. n Recharging unit and charging belt.
- Recharging unit and charging belt.
What is the Axonics Sacral Neuromodulation System?



This full system implant has a different patient programmer. It is simple in its working and affords patients the ability to make basic adjustments to the device.
- An implantable pulse generator (battery) which is inserted under the skin. Usually in the buttock area.
- An electrode or thin wire with barbs/tines that carries the electrical pulses to the bladder nerves.
- Recharging unit and charging belt.
This full system implant requires recharging approximately once every 6 months for approximately 70-90 minutes. The IPG has an estimated battery life of 15-20 years before it will need replacing.
Comparing the full system implants
| Feature | Interstim X (Medtronic) | Interstim Micro (Medtronic) | rSNM System (Axonics) |
|---|---|---|---|
Dimensions (mm) H x L x W | 44 x 51 x 7.7 | 17 x 47 x 5 | 22 x 42 x 6 |
| Volume (CC) | 12.5 | 2.8 | 5.5 |
| Weight (g) | 22 | 7.3 | 11 |
| Amplitude control | Constant current | Constant current | Constant current |
| MRI compatibility | Full body 1.5T + 3T | Full body 1.5T + 3T | Full body 1.5T + 3T |
| Appearance | Touchscreen smart phone | Touchscreen smart phone | Full colour LCD |
| Remote control | 
| 
| 
|
| Wireless range (ft) | 6.5 | 6.5 | 3 |
| Number of programmes stored | Up to 11 | Up to 11 | 2 |
| Charging position | N/A | Anywhere on device | On the rectangle |
| Charging frequency | NA | Once a week | Every 6 months |
| Duration | N/A | 30 minutes | 90 minutes |
During the procedure
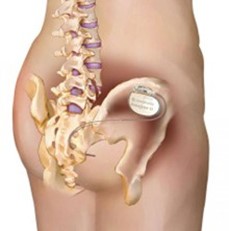
- You will come to theatre on the day of your procedure.
- The surgeon may draw/mark the ideal position of the nerve simulator. This will be on the upper buttock, below your belt line but high enough so you won’t sit on it. If you have a preference please let your surgeon know. If you have tattoos on your back there is a chance they will be damaged or distorted.
- The procedure will be done using local anaesthetic and sedation with you asleep on your front. The surgeon will use X-rays during the procedure to make sure the wire is placed in the correct position.
- The long-term electrode/wire will be inserted close to the nerves in your lower back.
- Once we are happy the electrode is in the correct position, it is then connected to the nerve stimulator which will be inserted under the skin in the upper buttock (image to the right).
- The scar will be about 5cm long and the stitches will be dissolvable.
After the procedure
You will probably feel sore and slightly uncomfortable at the site of your implant following surgery. This is completely normal and you can take painkillers. We also ask that you keep your dressings dry and clean for around 5-7 days after your procedure.
The stitches used are dissolvable and therefore do not need to be removed. It is important to keep the site clean and you will be sent home with antibiotics for 5-7 days to reduce the risk of infection.
Once at home, you can gradually increase your activity level as your incision heals. You may be aware of the nerve stimulator at first, but this should gradually reduce over time.
The stimulator has many different settings and we will programme the implant for you. We may be able to do this for you on the day of implantation, or we may need you to come back 1-2 weeks later.
Normally your SNM team will plan a hospital check 8 weeks after the implant to check everything is okay. The settings may need to be fine-tuned and sometimes several visits may be needed during the first few months.
After this we will stay in touch with you over the phone and/ or via face-to-face follow-ups. Occasionally we will ask you to complete bladder diaries and symptom questionnaires so that we can monitor your progress. You can contact us at any time if you are having any problems or if you need advice.
You will be given information on how to look after your permanent implant and how to use the your patient programmer.
What happens when the neurostimulator battery runs down?
If you have a rechargeable device you will need to fully charge the battery every week or when it runs low depending on the device. It should last for approximately 15-20 years before it needs replacing.
If you have decided on a recharge-free device this should last for approximately 12 years. If you feel a change in the stimulation, contact your SNM team so the battery can be checked.
If the battery has run down, the battery will need to be replaced, but the wire does not usually need to be changed. This requires a minor surgical procedure to remove the old battery and implant a new one.
What else do I need to know?
Medical procedures and equipment
Before you undergo medical tests or treatments; always tell your doctor or dentist that you have an implanted Sacral Neuromodulator along with the system type. Most medical procedures and routine tests, such as X-ray, should not affect your device. However, the following may adversely affect you and/or your device:
- Cardiac devices.
- Heart defibrillators.
- Lithotripsy (e.g. for kidney stones).
- Radiation therapy over the device.
- Radiofrequency (RF)/microwave ablation.
- Ultrasound scanning equipment over the device.
- Diathermy – electrocautery used during surgery. If you are having surgery then you need to tell your surgeon as they should avoid using monopolar diathermy and are required to use bipolar diathermy instead. Anyone who has an implanted neuromodulator (even if it is turned OFF) cannot have any shortwave diathermy, microwave diathermy or therapeutic ultrasound diathermy anywhere on their body.
- Magnetic resonance imaging (MRI) – all new full implant devices made after February 2020 are MRI safe however there are still set guidance which should be followed. If you have an Axonics device in place please contact your SNM team for a review before your MRI. If you have a Medtronic device then please make sure you switch your device into MRI SAFE MODE. This can be done on your handset.
Tell your doctor that you have an SNM device before you have tests like an ECG or EEG. The pulses from your system may interfere with the test.
Commercial equipment
Commercial electrical equipment (for example arc welders, induction furnaces) as well as high voltage power lines may interfere with your nerve stimulator system. You should discuss this with your employer if you work in an environment with these.
Theft detectors and screening devices
Airport screening systems or theft detectors found in shops, can cause the neurostimulator to turn OFF or ON. You would need to use your patient programmer to switch your neurostimulator back ON or OFF.
To avoid possible problems with airport screening systems, you may want to show an identification card / hospital letter and ask those in charge to let you bypass the screening device.
Possible complications
There are a few adverse events that have been reported as a result of the implant and the percentage of people they affect:
- Failure to improve symptoms (20%).
- Need for replacement or removal of implant (20%).
- Pain at the neurostimulator/battery site (15.5%).
- New pain (including leg or foot) (9%).
- Lead migration (moving inwards or outwards) (8.4%).
- Infection (6.1%).
- Transient electric shock (5.5%).
- Pain at lead site (5.4%).
- Adverse change in bowel function (3.0%).
- Technical problems (1.7%).
- Change in menstrual cycle (1%).
- Adverse change in voiding function (0.6%).
- Skin irritation (0.5%).
- Suspected nerve injury (0.5%).
- Device rejection (0.5%).
- Other (9.5%).
If any of these complications happen then you may need the device removed, or the wire or battery resited (moved) as it may stop working or it can cause you pain.
What do I use the patient programmer for?
The patient programmer is used for the following things:
- Show whether the stimulation is ON or OFF.
- Turn it ON or OFF.
- Show the level of stimulation and adjust it as required.
- Show whether the programmer batteries are low.
- In some cases to change the stimulation settings.
Frequently asked questions about the permanent system
Will people be able to see it?
No. The system is placed completely under your skin so others will not see it. They may see the scar you have depending on what swimsuit you wear or underwear you have on.
What do I do if the stimulation becomes uncomfortable?
If the stimulation becomes uncomfortable, use your patient programmer to decrease the stimulation level. If it is still uncomfortable please switch it OFF and contact your SNM team.
Will the stimulation keep me awake at night?
No, it should not. If it does, contact your SNM team.
Can SNM therapy be used during pregnancy?
There have not been any medical trials into the safety of SNM during pregnancy yet. If you think you are or might be pregnant, turn OFF your device and inform your SNM team.
Can I have sex after my SNM full system is implanted?
Yes. Sexual activity is not restricted but we recommend not having sex for about 2-4 weeks until the system has started to embed in scar tissue to reduce the risk of it moving.
Will a microwave oven interfere with the nerve stimulator?
Generally not. Most home appliances do not affect the way your nerve stimulator operates but do not keep your charging unit too close to it for long periods of time.
Will the SNM system limit my activities?
Normally there are no restrictions to physical activities. You should avoid activities that involve sudden, excessive or repetitive bending, twisting, bouncing or stretching soon after the surgery. These movements could damage or move the wire making it less effective.
May I dive after my SNM nerve stimulator is implanted?
Yes, but do not dive below 10 meters of water or enter hyperbaric chambers above 202.65 kilopascals (kPa), (2.0
ATA ). Before diving or using a hyperbaric chamber, discuss the effects of high pressure with your SNM team. The main concern is that the high pressure may cause the battery to blow up.
Are the sensations felt during the test different than with the permanent implant?
The sensations should be the same or similar.
Should I turn the neurostimulator OFF to go to the toilet?
This is not usually necessary.
Research at Bristol Urological Institute
Delivering and participating in research is a key objective of Bristol Urological Institute. As a patient receiving care in our department, we provide opportunities for you to be an active participant in research, which improves treatments and patient outcomes.
You can choose whether you want to take part in research. For information about opting out speak to your clinician or visit:
Patient Data & Research Privacy Policy | North Bristol NHS Trust
References and further information
© North Bristol NHS Trust. This edition published May 2024. Review due May 2027. NBT003133.
Support your local hospital charity

See the impact we make across our hospitals and how you can be a part of it.

